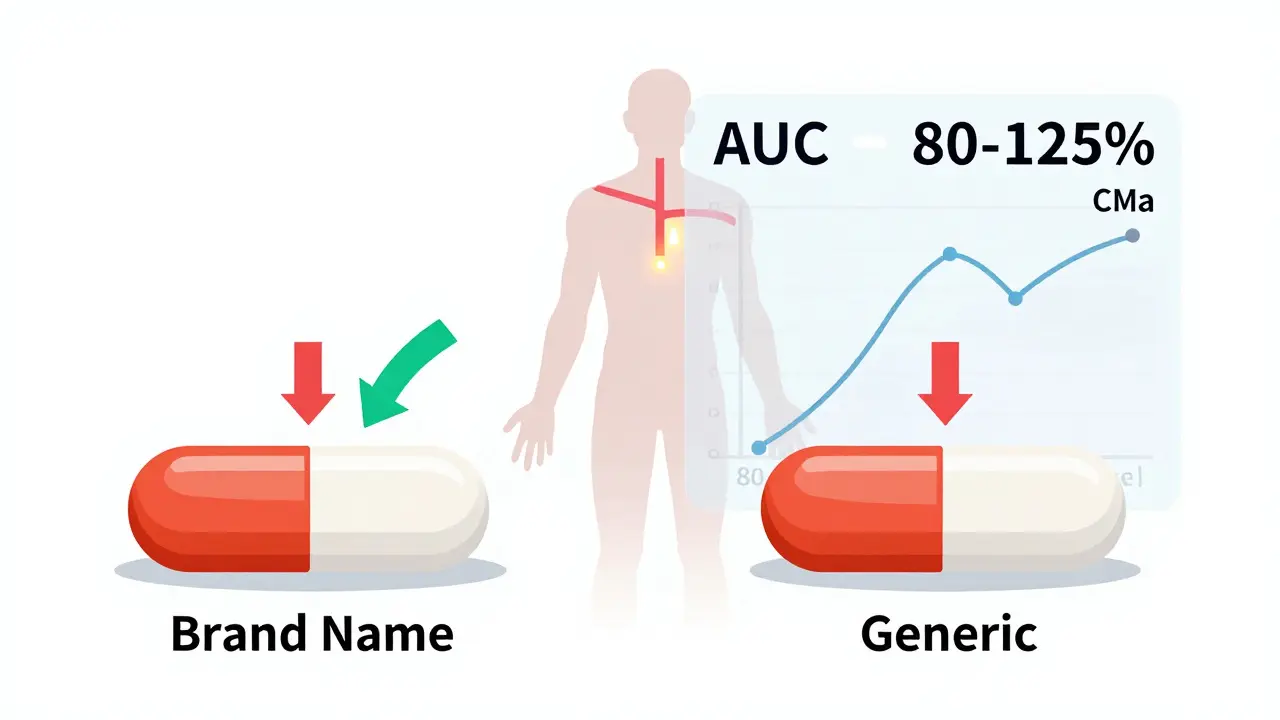
Bioequivalence studies are the scientific foundation that ensures generic drugs work the same as brand-name versions. The FDA requires strict pharmacokinetic testing to prove identical absorption and effectiveness - a process that keeps patients safe and lowers drug costs.

