Genetic Factors That Increase Susceptibility to Drug Side Effects
 Dec, 1 2025
Dec, 1 2025
Drug-Gene Interaction Checker
Enter a medication name to check for known genetic interactions that could affect how it works for you.
Disclaimer: This tool provides educational information only. It is not a substitute for professional medical advice.
Results are based on known pharmacogenetic interactions. They do not guarantee a reaction will occur, but indicate increased risk.
Some people take a medication and feel fine. Others get sick-sometimes dangerously so-even when they take the exact same dose. It’s not always about dosage, allergies, or lifestyle. Often, it’s written in their DNA. Genetic differences can turn a safe drug into a dangerous one, or make a common side effect unbearable. This isn’t rare. It’s happening every day in clinics, hospitals, and homes across the country.
Why Do Some People React Worse to Medications?
It comes down to how your body processes drugs. Your genes control the enzymes that break down medications, the transporters that move them in and out of cells, and the receptors they bind to. If any of these are slightly off because of your DNA, the drug can build up to toxic levels, disappear too fast to work, or trigger an immune reaction.
Take CYP2D6, one of the most important drug-metabolizing enzymes. About 7% of people are poor metabolizers-they break down drugs like codeine, tamoxifen, and some antidepressants extremely slowly. That means the drug lingers, increasing side effects. On the flip side, ultrarapid metabolizers process these drugs so fast they turn codeine into morphine at five to fifty times the normal rate. In breastfeeding mothers, this can lead to fatal respiratory depression in infants. The FDA added a black box warning to codeine labels in 2013 because of this exact risk.
Genes That Can Turn a Drug Deadly
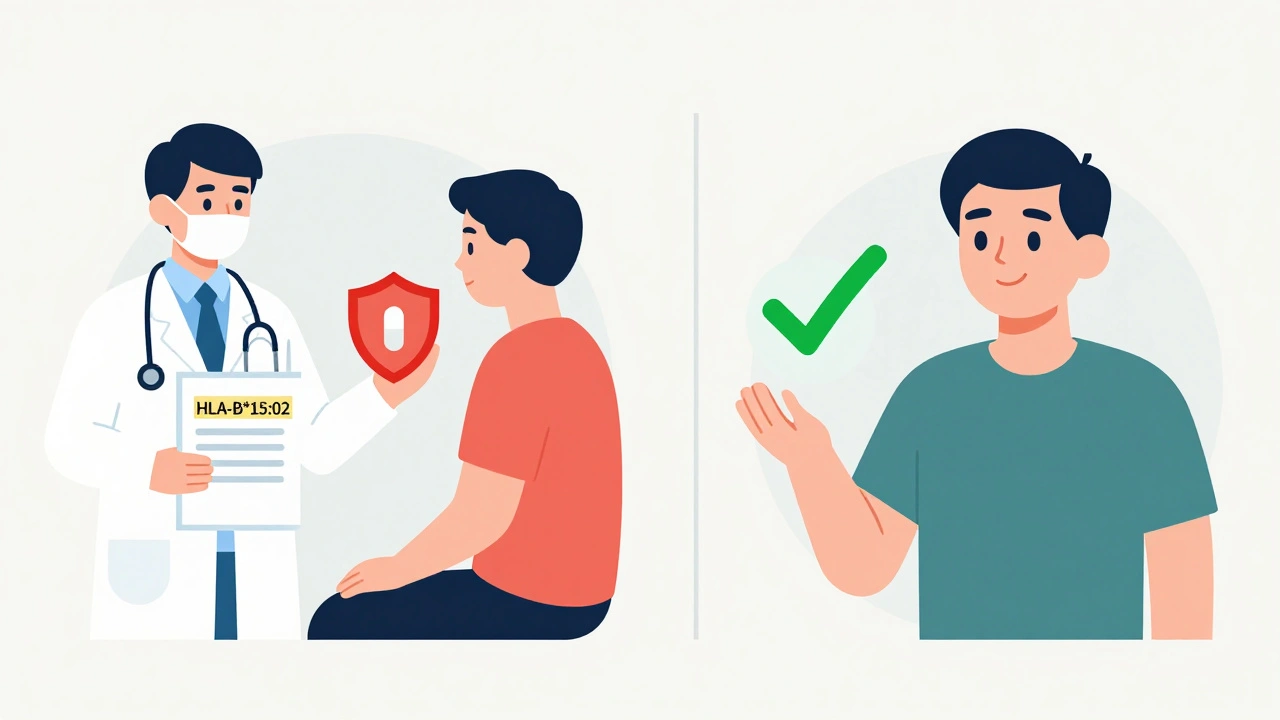
Not all genetic risks are subtle. Some are life-or-death. The HLA-B*15:02 gene variant is a prime example. People with this variant, especially those of Southeast Asian descent, face a 100 to 150 times higher risk of developing Stevens-Johnson Syndrome or toxic epidermal necrolysis if they take carbamazepine or phenytoin-common drugs for epilepsy and nerve pain. These conditions destroy the skin and mucous membranes. The FDA requires testing for this variant before prescribing these drugs in high-risk populations. Skip the test, and you risk hospitalization-or death.
Another dangerous combo is HLA-B*57:01 and abacavir, an HIV drug. Carriers of this variant have a 50% chance of a severe hypersensitivity reaction, including fever, rash, and organ failure. But here’s the twist: if you test negative for HLA-B*57:01, your risk drops to less than 1%. That’s one of the clearest examples of genetic testing preventing harm. Today, every patient starting abacavir gets tested first. It’s standard. And it works.
Warfarin and the Genetics of Blood Thinning
Warfarin is a classic case of how genetics can make dosing a guessing game. It’s used to prevent strokes and clots, but it’s notoriously hard to dose correctly. Too little, and you’re at risk of clots. Too much, and you bleed internally. For decades, doctors relied on trial and error-weeks of blood tests, adjusting doses, watching for bruising.
Then came genetics. Two genes-VKORC1 and CYP2C9-explain up to 40% of why people need different warfarin doses. People with certain variants of VKORC1 need much lower doses. Those with CYP2C9*3 variants break down warfarin slowly, so even small doses can be dangerous. The FDA now lists these genes on the drug label. Some hospitals use genetic tests to start patients on the right dose from day one, cutting the time to reach a safe level from weeks to days.
Cardiac Drugs and Hidden Heart Risks
Some drugs cause dangerous heart rhythms-like torsades de pointes-that can lead to sudden death. In about 5% of these cases, the patient has an undiagnosed inherited form of Long QT Syndrome. Mutations in genes like KCNQ1, KCNH2, and SCN5A make the heart’s electrical system unstable. A common antibiotic, antipsychotic, or even an antihistamine can trigger a fatal arrhythmia in these people.
Even more surprising: researchers found that 2.2% of people who had severe drug-induced QT prolongation had a rare variant in the ANK2 gene. This gene isn’t even a heart ion channel-it’s a structural protein. But when mutated, it makes the heart extra sensitive to drugs. That’s why genetic testing isn’t just about metabolism anymore. It’s about hidden vulnerabilities.
Why Genetic Testing Isn’t Everywhere Yet
Despite all this, most people still get medications without any genetic screening. Why? For one, it’s not always practical. Testing costs $250 to $500, and insurance doesn’t always cover it. Medicare Advantage plans only cover preemptive testing for 28% of patients. Many doctors don’t know how to interpret the results. A 2023 survey found that nearly 70% of physicians felt untrained to use pharmacogenetic data.
Another issue: not all genetic links are strong. For example, the CYP2D6 gene affects dozens of drugs, but only a few have clear clinical guidelines. And even when a gene variant increases risk, it doesn’t guarantee a reaction. Only 5-10% of people with HLA-B*57:01 actually develop abacavir hypersensitivity. But because the risk is so high when it happens, testing is still worth it.
Implementation is also messy. Hospitals need to integrate genetic data into electronic health records. That takes months, millions of dollars, and trained pharmacists. Only 37% of U.S. hospitals have clinical decision support for pharmacogenetics. At Mayo Clinic, their preemptive genotyping program reduced hospitalizations from drug side effects by 23%. But they had to hire dedicated pharmacogenetics staff and redesign workflows.
What’s Changing-and What’s Next
The tide is turning. The FDA now requires pharmacogenetic information on the labels of 18 drugs, up from just 3 in 2010. In 2023, 32% of new drugs included genetic testing guidance. The European Medicines Agency and Japan’s drug regulators have similar rules. The All of Us Research Program has already returned genetic results to over 200,000 Americans, finding that 42% carry at least one actionable variant.
Future tools are getting smarter. Instead of looking at one gene at a time, scientists are building polygenic risk scores-combining dozens of small genetic signals to predict side effect risk. A 2024 study showed a 15-gene score could predict statin-induced muscle damage with 82% accuracy, far better than checking just SLCO1B1 alone.
And soon, whole-genome sequencing may become routine. A 2023 study found that sequencing identified actionable drug-gene interactions in 91% of participants. That means one test could guide your medication use for life.
Real Stories, Real Impact
At Vanderbilt’s PREDICT program, 65% of doctors said genetic results changed how they prescribed drugs. One patient avoided severe nausea because her CYP2D6 status was checked before tamoxifen. Another avoided a dangerous drug interaction after her pharmacist flagged a CYP2C19 poor metabolizer result.
But it’s not perfect. Some patients get false positives. Others wait weeks for results. One woman in the U.K. was denied carbamazepine after a false HLA-B*15:02 result-delaying treatment for her seizures. These errors are rare, but they matter.
Genetic testing isn’t magic. It’s a tool. And like any tool, it works best when used correctly.
What You Can Do
If you’ve had bad side effects from a medication, or if a family member has, talk to your doctor. Ask: Could my genes be playing a role? If you’re starting a new drug-especially one for depression, epilepsy, heart disease, or cancer-ask if genetic testing is recommended.
Don’t rely on direct-to-consumer tests like 23andMe for medical decisions. Many of their pharmacogenetic reports aren’t validated for clinical use. The FDA has issued warnings to companies that overstate their results.
Instead, ask your doctor about testing through a clinical lab with pharmacogenetics expertise. If your hospital has a pharmacogenomics program, sign up. If not, push for it.
Genetics isn’t about destiny. It’s about safety. The right drug, at the right dose, for your body-that’s the goal. And now, more than ever, we have the tools to get there.
Can genetic testing prevent all drug side effects?
No, genetic testing can’t prevent all side effects. It only helps with those caused by known gene-drug interactions. Many side effects come from age, other medications, liver or kidney function, or lifestyle factors. But for certain high-risk drugs-like warfarin, carbamazepine, or abacavir-genetic testing can prevent serious, even fatal, reactions. It’s not a full solution, but it’s a powerful tool for specific cases.
How much does pharmacogenetic testing cost?
Comprehensive pharmacogenetic tests typically cost between $249 and $499. Companies like Color Genomics, OneOme, and 23andMe Health + Ancestry offer them. Insurance coverage varies widely: Medicare Advantage plans cover preemptive testing for only 28% of beneficiaries, and many private insurers require prior authorization. Some hospitals offer testing at no cost as part of research programs or clinical initiatives.
Are direct-to-consumer genetic tests reliable for drug safety?
Most are not. Companies like 23andMe provide genetic reports, but many of their pharmacogenetic results aren’t clinically validated. The FDA has issued 12 warning letters to genetic testing companies for overstating the accuracy or clinical utility of their drug-related results. Always consult a healthcare provider or pharmacist before making medication changes based on a consumer test.
Which drugs currently require genetic testing before use?
The FDA lists 18 drugs with mandatory or strongly recommended genetic testing. These include abacavir (HLA-B*57:01), carbamazepine (HLA-B*15:02), warfarin (CYP2C9/VKORC1), clopidogrel (CYP2C19), and codeine (CYP2D6). Many others, like tamoxifen, statins, and certain antidepressants, have strong genetic guidance but aren’t legally required. The list is growing-32% of new drugs approved in 2023 included pharmacogenetic information.
Is pharmacogenetic testing covered by insurance?
It depends. Medicare covers testing for only 7 of the 128 gene-drug pairs recognized by the FDA. Private insurers vary widely. Some cover testing for specific high-risk drugs like abacavir or warfarin, especially if ordered by a specialist. Others require prior authorization or only cover it if you’ve already had a side effect. Coverage is improving slowly, but out-of-pocket costs are still common.
Can I get tested before I need a drug, or only after a side effect?
You can-and should-get tested before you need a drug. Preemptive testing means one test can guide your care for years. Hospitals like Mayo Clinic and Vanderbilt test patients for 10-12 key genes before they’re ever prescribed a medication. The results are stored in their medical records and used whenever a new drug is considered. This approach has been shown to reduce adverse drug reactions by up to 33% in children and 23% in adults.
Why don’t all doctors use genetic testing?
Many doctors haven’t been trained to use it. A 2023 survey found that 68.5% of physicians felt insufficiently trained to interpret pharmacogenetic results. Others face workflow barriers-EHR systems don’t integrate genetic data well, results take days to return, and there’s no clear protocol for when to test. Even when guidelines exist, they’re often buried in clinical notes. Change is happening, but slowly.
Are certain ethnic groups more at risk for genetic drug reactions?
Yes. HLA-B*15:02 is common in people of Han Chinese, Thai, and Malaysian descent, making them far more vulnerable to carbamazepine reactions. CYP2D6 ultrarapid metabolizers are more common in North African and Middle Eastern populations. But here’s the problem: 95% of pharmacogenetic research has been done in people of European ancestry. This means we don’t fully understand risks in African, Indigenous, or South Asian populations-despite higher genetic diversity. That’s a major gap in patient safety.

Ignacio Pacheco
December 3, 2025 AT 20:42Kidar Saleh
December 3, 2025 AT 23:10Chloe Madison
December 5, 2025 AT 19:11Vincent Soldja
December 6, 2025 AT 07:15Makenzie Keely
December 7, 2025 AT 16:25Francine Phillips
December 8, 2025 AT 07:42Katherine Gianelli
December 8, 2025 AT 11:55Joykrishna Banerjee
December 10, 2025 AT 03:46Myson Jones
December 11, 2025 AT 04:05parth pandya
December 11, 2025 AT 10:17Albert Essel
December 12, 2025 AT 11:05Steve Enck
December 12, 2025 AT 16:58